Answer:
increase the concentration of NiCO4
withdraw CO from the products as it is forming
Step-by-step explanation:
According to Le Chatelier's principle when a system at equilibrium is subjected to changes in temperature, pressure or concentration the equilibrium will shift in a direction to undo the effect of the induced change.
The given reaction is:
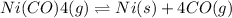
In order to increase the amount of Ni, two approaches can be adopted
1) Increasing the concentration of Ni(CO)4 will shift the equilibrium in a direction that would enhance its consumption i.e. towards the right
2) Withdrawing CO from the reaction will shift the equilibrium in a direction that would increase its production i.e. to the right