Start by writing the atoms balance:
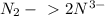
Now, determine the change of oxidation states:
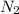
has oxidation state 0, so each N has to gain 3 electrons to become
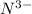
.
That, means that you need 6 electrons to balance the charges, resulting in:
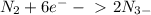 And the answer is 6 mole of electrons.
And the answer is 6 mole of electrons.