Answer: The name of the compound sodium oxide.
Explanation: Sodium is Na and its atomic mass is 22.99 amu. Oxygen is O and its mass is 16.00 amu. Na is a first group metal with one valence electron where as oxygen is a non metal with six valence electrons. Na loses one electron to get its nearest noble gas configuration and oxygen accepts two electrons to get its nearest noble gas configuration. So, the charge for Na is +1 and the charge for O is -2.
For each oxygen atom, two atoms of sodium are required. Hence, formula of the compound is
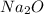 and its name is Sodium oxide.
and its name is Sodium oxide.
Formula mass = [2(22.99)+16.00]amu
= (45.98 + 16.00)amu
= 61.98 amu