Answer : The number of moles of ammonia gas is, 2.232 moles
Explanation : Given,
Volume of ammonia gas = 50 L
As we know that at STP,
1 mole of gas contains 22.4 L volume of gas.
As, 22.4 L volume of ammonia gas present in 1 mole of ammonia gas
So, 50 L volume of ammonia gas present in
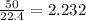 mole of ammonia gas
mole of ammonia gas
Therefore, the number of moles of ammonia gas is, 2.232 moles