Answer: tetrahedral
Step-by-step explanation:
Hybridization is calculated using the Lewis dot structures of all the compounds.
Formula used to calculate the number of atomic orbitals around central metal atom is:
Number of atomic orbitals around central metal atom = Number of bond pairs + Number of lone pairs
Bond pairs for a double bond and triple bond is taken as 1 only.
Given: Number of bond pairs = 4
Number of lone pairs = 0
If Number of atomic orbitals around central metal atom are 4 , the hybridization is
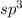 and electron domain geometry is tetrahedral.
and electron domain geometry is tetrahedral.