Answer: 7.6% KCl
Explanation: Mass of solute is given as 13.6 grams and the mass of solution is 180 grams. The question asks to calculate the solution concentration, expressed as a percent by mass.
The formula for percent by mass is:
% by mass =
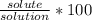
Let's plug in the values in the formula and do the calculations.
% by mass =
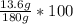 = 7.6%
= 7.6%
So, the correct choice is C) 7.6% KCl.