For the first equation, we know that the Be gained a proton and became B. In order to cancel this out, we would need a
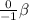
particle. This particle is also called a beta particle.
For the second equation, we know that Si gained a proton and became P. The particle for this equation would also be a beta particle (
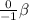
), as the same thing is happening as in the first equation.
For the third equation, we know that some atom plus an alpha particle gives us platinum-192. By subtracting the 4 and 2 from the alpha particle, we get the missing atom to be
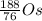
, or osmium-188.
For the last equation, we can simply add the mathematical value of the beta particle to the aluminum-28. This gives us a changed proton number, but not atomic mass with the missing atom being
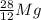
, or magnesium-28.
Hope this helps!