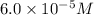Answer : The correct option is,
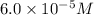
Explanation : Given,
pOH = 4.22
pOH : It is defined as the negative logarithm of hydroxide ion concentration.
Now we have to calculate the
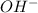 concentration.
concentration.
Formula used :
![pOH=-\log [OH^-]](https://img.qammunity.org/2018/formulas/chemistry/high-school/tyqnxkdounrtow0llesefga8x48nlscnj6.png)
Now put all the given values in this formula, we get the concentration of hydroxide ion.
![4.22=-\log [OH^-]](https://img.qammunity.org/2018/formulas/chemistry/high-school/xchq5bc2y79rfckn6qfntx50zvb1gv4cmm.png)
![[OH^-]=6.0* 10^(-5)M](https://img.qammunity.org/2018/formulas/chemistry/high-school/eth15cv14gu6s0st0q21xps1wa5x77b0yd.png)
Therefore, the
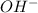 concentration is,
concentration is,