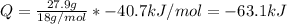Answer:
Quantity of heat released = -63.1 kJ
Step-by-step explanation:
Given:
Enthalpy of vaporization of water, ΔHvap = 40.7 kJ/mol
Mass of water, m = 27.9 g
To determine:
The amount of heat (Q) released
Step-by-step explanation:
The reaction is: H2O(g) ↔ H2O(l)
The amount of heat evolved during condensation which involves a phase transition from vapor to liquid is given as:
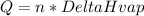
n = moles of water
Since this is a condensation process: ΔHcond = -ΔHvap