Answer:
The enthalpy change when 2.50 mol of nitrogen dioxide decomposes is 84.6 kJ/mol.
Step-by-step explanation:
Enthalpy of the formation of
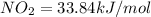
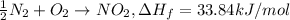
Enthalpy of decomposition when one mol of nitrogen-dioxide decomposes.
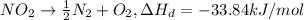
When 1 mol of
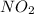 decomposes it gives = -33.84 kJ/mol
decomposes it gives = -33.84 kJ/mol
When 2.50 mol of nitrogen-dioxide decomposes to give:
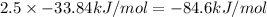
The enthalpy change when 2.50 mol of nitrogen dioxide decomposes is 84.6 kJ/mol.