Answer : The percentage by mass of water in the hydrate sample is, 51.16%
Explanation :
First we have to calculate the mass of water.
Mass of water = Mass of hydrated magnesium sulfate - Mass of sample after heating
Mass of water = 4.052 - 1.979 = 2.073 g
Now we have to calculate the percentage by mass of water in the sample.
Formula used :
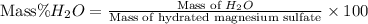
Now put all the given values in this formula, we get
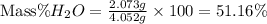
Therefore, the percentage by mass of water in the hydrate sample is, 51.16%