We are given –
- Mass of C₁₂H₂₂O₁₁ is 179g. So, let's find molar mass and moles number of C₁₂H₂₂O₁₁.

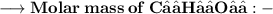

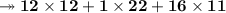

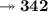
 ____________________
____________________
Calculating the number of moles present in 179 g of C₁₂H₂₂O₁₁.

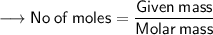

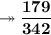

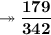

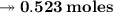
- Avogadro number = 6.022×10²³
☀️No.Of Molecules –
=Moles × Avogadro number
= 0.523 × ( 6.02×10²³)
= 3.15 × 10²³
_________________________________