Step-by-step explanation:
The given data is as follows.
Mass of
 = 1.15 g
= 1.15 g
Mass of
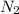 = 1.55 g
= 1.55 g
Therefore, moles of oxygen present will be as follows.
No. of moles of
 =
=
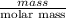
=
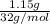
= 0.035 mol
No. of moles of
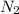 =
=
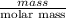
=
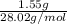
= 0.055 mol
Hence, total no. of moles = moles of
 + moles of
+ moles of
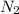
= (0.035 + 0.055) mol
= 0.09 mol
Now, it is known that at STP volume is 22.4 L/mol. Hence, volume of the gas sample at STP for 0.09 moles will be as follows.
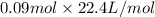
= 2.01 L
Thus, we can conclude that volume of the given gas sample is 2.01 L.