Answer : The enthalpy of the reaction = -1839.6 KJ
Solution : Given,
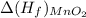 = -520.0 KJ/mole
= -520.0 KJ/mole
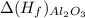 = -1699.8 KJ/mole
= -1699.8 KJ/mole
The balanced chemical reaction is,
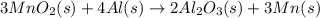
Formula used :

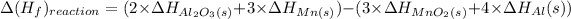
We know that the standard enthalpy of formation of the element is equal to Zero.
Therefore, the enthalpy of formation of (Mn) and (Al) is equal to zero.
Now, put all the values in above formula, we get
![\Delta (H_(f))_(reaction)=[2moles* (-1699.8 KJ/mole)}+3moles* (0\text{ KJ/mole}})]-[(3moles*(-520.0KJ/mole }+4moles*(0\text{ KJ/mole})]](https://img.qammunity.org/2018/formulas/chemistry/high-school/fw7z286t7j2vibxf22ffhsb6osjeswkwoi.png)
= (-3399.6) + (1560)
= -1839.6 KJ