Answer:- C.
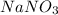 .
.
Explanations:- From solubility curve, the solubility of sodium chloride at 0 degree C is around 36 g per 100 g of water.
Solubility of potassium bromide at 0 degree C is 60 g per 100 g of water.
solubility of sodium nitrate at 0 degree C is 70 g per 100 g of water and the solubility of potassium nitrate at 0 degree C is 13 g per 100 g of water.
Since the solubility of sodium nitrate is highest, the right choice is C.
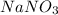 .
.