Answer: Catalyst
Step-by-step explanation:
A catalyst is a substance which increases the rate of a reaction by taking the reaction through a different path which has a lower activation energy .Thus more reactant molecules can cross the energy barrier and convert to products.
The catalyst itself does not take part in the chemical reaction and is regenerated as such at the end.
Catalyst does not affect the position of equilibrium.
For example:
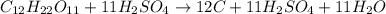
 acts as a catalyst here as it gets regenerated as such.
acts as a catalyst here as it gets regenerated as such.