Answer:
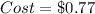
Step-by-step explanation:
Hello,
At first, the ionic neutralization chemical reaction turns out into:
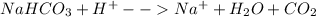
In such a way, we develop the stoichiometry calculation to obtain the kilograms of sodium bicarbonate:

Now, we compute the cost by considering the neutralized kilograms of sodium bicarbonate as shown below:
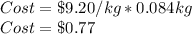
Best regards.