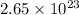Answer : The number of atoms of zinc metal are,
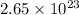
Explanation : Given,
Moles of zinc metal = 0.44 mole
As we know that, 1 mole of substance contains
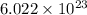 number of atoms.
number of atoms.
As, 1 mole of zinc metal contains
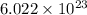 number of atoms.
number of atoms.
So, 0.44 mole of zinc metal contains
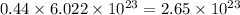 number of atoms.
number of atoms.
Therefore, the number of atoms of zinc metal are,