Answer: Option (c) is the correct answer.
Step-by-step explanation:
A hydrocarbon that contains only carbon and hydrogen atoms attached through single bonds is known as an alkane.
General formula for alkanes is
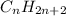 where n is any integer.
where n is any integer.
For example, a hydrocarbon with 30 hydrogen atoms will have the value of "n" as follows.
Total hydrogen atoms = 2n + 2
30 = 2n + 2
30 - 2 = 2n
n = 14
Therefore, number of carbon atoms in this hydrocarbon will be 14.
Hence, we can conclude that formula for an alkane which contains 30 hydrogen atoms is
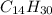 .
.