Answer: Option (B) is the correct answer.
Step-by-step explanation:
A molecular compound is defined as the compound in which combining atoms are chemically combined together by sharing of electrons.
This means a covalent bond will be present between the atoms of a molecular compound.
And, a covalent bond is always formed by non-metals.
Therefore,
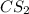 ,
,
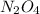 and
and
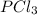 are all molecular compounds as all of them have a covalent bond.
are all molecular compounds as all of them have a covalent bond.
But in
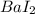 , a chemical bond is formed between a metal and a non-metal. Hence, it is not a molecular compound.
, a chemical bond is formed between a metal and a non-metal. Hence, it is not a molecular compound.
Thus, we can conclude that out of the given options
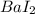 does not represent a molecular compound.
does not represent a molecular compound.