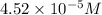Answer : The correct option is,
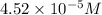
Explanation :
The balanced equilibrium reaction will be,
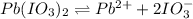
The expression for solubility constant for this reaction will be,
![K_(sp)=[Pb^(2+)][IO_3^-]^2](https://img.qammunity.org/2018/formulas/chemistry/high-school/fjwg3xjazf9yrgfw65pyf3kwgkjiakn24t.png)
Let the solubility will be, 's'
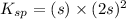
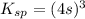
Now put the value of
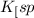 in this expression, we get the solubility of Lead(II) iodate.
in this expression, we get the solubility of Lead(II) iodate.
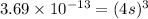
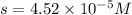
Therefore, the solubility of Lead(II) iodate is,