Answer : The half-life of the decomposition is, 0.203 seconds.
Solution :
Half-life period of a first order reaction is defined as the the time taken for any fraction of the reaction to complete is independent of the initial concentration.
Formula of the half-life period of a first order reaction will be,
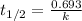
where,
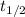 = half-life of the reaction
= half-life of the reaction
k = rate constant =
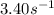
Now put all the values in this formula, we get the half-life of the reaction.
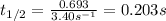
Therefore, the half-life of the decomposition is, 0.203 seconds.