Answer:

Explanation:
Molecular formula is the chemical formula which depicts the actual number of atoms of each element present in the compound.
Sulphuric acid is an acid which dissociates in water to give
 ions and has the molecular formula of
ions and has the molecular formula of

a.
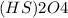 has 2 atoms of hydrogen, two atoms of sulfur and four oxygen atoms.
has 2 atoms of hydrogen, two atoms of sulfur and four oxygen atoms.
b.
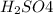 has 2 atoms of hydrogen, one atom of sulfur and four oxygen atoms.
has 2 atoms of hydrogen, one atom of sulfur and four oxygen atoms.
c.
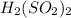 has 2 atoms of hydrogen, two atoms of sulfur and four oxygen atoms.
has 2 atoms of hydrogen, two atoms of sulfur and four oxygen atoms.
d.
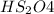 has 1 atom of hydrogen, two atoms of sulphur and four oxygen atoms.
has 1 atom of hydrogen, two atoms of sulphur and four oxygen atoms.