Answer : The relationship between a mole and the Avogadro number is, 1 mole of a substance contains
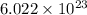 number of atoms, molecules or ions.
number of atoms, molecules or ions.
Explanation :
Mole : It is defined as the amount of substance that contain the same number of units as there are atoms present in exactly 12 grams of carbon-12.
Avogadro's number : It is defined as the number of atoms molecules present in a mole of any substance.
The Avogadro's number is represented as,
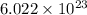
The relationship between a mole and Avogadro number are,
As, 1 mole of a substance contains
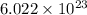 number of atoms, molecules or ions.
number of atoms, molecules or ions.