Answer : The concentration of final solution is, 0.197 M and the final volume of solution is, 400 ml
Solution :
First we have to calculate the final volume of solution.
Final volume of solution = Volume of
 + Volume of water added
+ Volume of water added
Final volume of solution = 225.0 ml + 175.0 ml = 400 ml
Now we have to calculate the concentration of final solution.
Formula used :
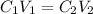
where,
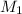 = concentration of
= concentration of
 solution = 0.350 M
solution = 0.350 M
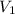 = volume of
= volume of
 solution = 225.0 ml
solution = 225.0 ml
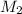 = concentration of final solution = ?
= concentration of final solution = ?
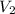 = volume of final solution = 400 ml
= volume of final solution = 400 ml
Now put all the given values in the above formula, we get the concentration of final solution.
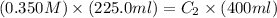
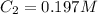
Therefore, the concentration of final solution is, 0.197 M