We are going to assume that the gas behaves like an ideal gas.
1) When we work at constant pressure (Isobaric) and the temperature increases we add more energy to the gas. The molecules will move faster causing the volume to increase. So if we increase the temperature, the volume increases, that is, we have a directly proportional relationship. We can apply Charles's Law:
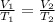
2) At constant volume (Isometric), if the temperature decreases there will be less energy in the system. Molecules move slower and will exert less pressure. So, we have a directly proportional relationship. We can apply Gay-Lussac's Law:
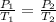
3) At constant temperature8Isothermic), if the volume decreases, it means that the molecules will be closer together, therefore they will exert more pressure and the pressure increases. It will be an inversely proportional relationship. We can apply Boyle's Law:
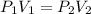
Therefore, in summary, the answers will be:
1) Increase - Direct
2) Decrease - Direct
3)Increase - Inverse