Answer:
The state of bromine is liquid at room temperature.
Step-by-step explanation:
We are given that room temperature is around
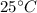
We have to find out the state of bromine at room temperature at
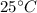
We change celsius into kelvin by the formula
kelvin measure= 273+ celsius measure
Therefore
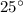 C= 273+25=298 k
C= 273+25=298 k
The attraction forces between the molecules of bromine is dispersion force.The dispersion force is the weakest intermolecular forces. it is a temporary attractive force.The molecules of bromine hold together with strong dispersion forces. Therefore, room temperature is not sufficient to overcome the strong dispersion forces . It requires more energy to break the substance . Therefore, the state of bromine is liquid at room temperature.
Answer:The state of bromine is liquid at room temperature.