It is given that that initial volume of gas is 20 cubic inches.
The initial pressure is given as 5 psi.
The final pressure is given as 10 psi
We are asked to calculate the final volume.
Let initial and final volume of gas is denoted as
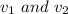
Let the initial and final pressure of gas is denoted as
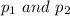
As per the question the temperature of a gas is constant.
From Boyle's law we know that pressure of a given mass of a gas is inversely proportional to the applied volume at constant temperature.
Hence mathematically
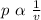
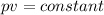
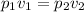
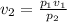
Putting the values of these respective quantities as mentioned above we get-
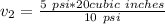
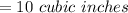 [ans]
[ans]