A. The radioactive decay equation is N = N0
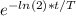
where T is the half-life (5730 years), N0 is the number of atoms at time t = 0 and N is the number at time t.
Rewriting this as:
(N/N0) =
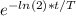
Since N = (1/8) N0 and substituting known values:
1/8 =
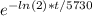
Taking ln of both sides:
ln(1/8)= -ln(2)*t/5730
t = - 5730 * ln(1/8) / ln (2)
t = 17,190 years
The tree was cut down 17,190 years ago.
B. N0 = 1,500,000 carbon-14 atoms
Since N = (1/8) N0
N = 187,500 carbon atoms left