Answer : The number of atoms of nitrogen present in
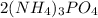 are, 6.
are, 6.
Explanation :
The given compound is,
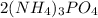
The chemical name of this compound is, ammonium phosphate.
In this compound,
The number of nitrogen atoms = 6
The number of hydrogen atoms = 12
The number of phosphorous atoms = 2
The number of oxygen atoms = 8
Hence, the number of atoms of nitrogen present in
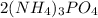 are, 6.
are, 6.