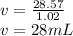Answer:
28 mL
Step-by-step explanation:
We want to know what volume of seawater contains 1.0 g of sodium.
We know that the total amount of water mass always 3.5% corresponds to the mass of sodium.
So if the total mass of sodium is 1.0g this is equivalent to 3.5% of 100% of the mass
Calculate 100% of the mass with a simple rule of three
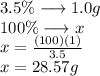
The total mass of seawater containing 1 g of sodium is 28.57 g
Now we calculate the volume that corresponds to this mass with the density
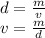
replace the values in the formula