Answer: The correct answer is 8.
Explanation: For the following equation:
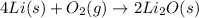
As oxygen gas is in excess, so lithium metal is considered as the limiting reagent because it limits the formation of product.
By Stoichiometry,
2 moles of lithium oxide is produced by 4 moles of Lithium
So, 4 moles of lithium oxide will be produced by =
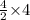 = 8 moles of lithium metal.
= 8 moles of lithium metal.
So, the correct answer is 8.