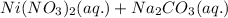Answer: The pair undergoing a precipitation reaction is
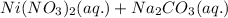
Step-by-step explanation:
Precipitation reaction is defined as the reaction in which an insoluble salt is formed when two solutions are mixed containing soluble substances. The insoluble salt settles down at the bottom of the reaction mixture.
For the given options:
Option 1:
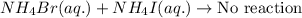
As, the cation is same in both of the reactants. Thus, the reactants will remain as such. Thus, no precipitation reaction occurs.
Option 2:
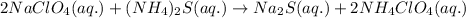
As, no solid product is getting formed. Thus, no precipitation reaction occurs.
Option 3:
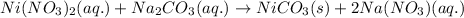
As, nickel (II) carbonate is getting formed as a solid. It is considered as a precipitate. Thus, it is a precipitation reaction
Option 4:
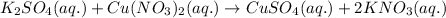
As, no solid product is getting formed. Thus, no precipitation reaction occurs.
Hence, the pair undergoing a precipitation reaction is