Answer: The rate of the forward reaction and the rate of the reverse reaction will be equal.
Step-by-step explanation:
The reactions which do not go on completion and in which the reactant forms product and the products goes back to the reactants simultaneously are known as equilibrium reactions.
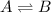
Equilibrium state is the state when reactants and products are present but the concentrations does not change with time.
For a chemical equilibrium reaction, dynamic equilibrium state means the rate of forward reaction is equal to the rate of the backward reaction. It is always achieved in a closed container so that no substance can leave the system.