Answer : The partial pressure of
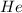 is, 270 Kpa
is, 270 Kpa
Solution :
According to the Dalton's law, the total pressure of the gas is equal to the sum of the partial pressure of the mixture of gasses.
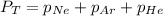
where,
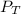 = total partial pressure = 750 Kpa
= total partial pressure = 750 Kpa
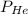 = partial pressure of helium = ?
= partial pressure of helium = ?
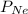 = partial pressure of neon = 230 Kpa
= partial pressure of neon = 230 Kpa
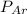 = partial pressure of argon = 250 Kpa
= partial pressure of argon = 250 Kpa
Now put all the given values is expression, we get the partial pressure of the helium gas.
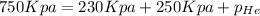
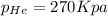
Therefore, the partial pressure of
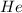 is, 270 Kpa
is, 270 Kpa