Answer:
We have the following reaction:
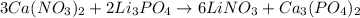
The first thing we have to do is check if the equation is balanced. For this we count the number of atoms of each element on each side of the reaction:
Ca: 3
N: 6
O: 26
Li: 6
P:2
As we can see the equation is balanced.
Now we calculate how many moles of Li3PO4 we would need if 3.4 moles of Ca(NO3)2 react:
According to the balanced equation for every 3 moles of Ca(NO3)2 that react we need 2 moles of Li3PO4.
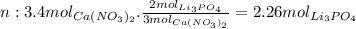
So we need 2.26 mol of Li3PO4, but we have 2.4. So the excess reactant is Li3PO4.
The correct answer then is option D: Li3PO4