Answer: 0.19 atm
Step-by-step explanation:
According to Dalton's law, the total pressure is the sum of individual pressures.
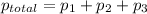
Given :
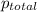 = 1.25 atm
= 1.25 atm
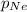 = 0.68 atm
= 0.68 atm
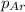 = 0.38 atm
= 0.38 atm
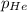 = ?
= ?

Putting in the values:
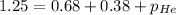
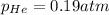
Thus partial pressure of the third gas helium is 0.19 atm