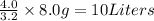Answer: 10 L
Step-by-step explanation:
Given : 3.2 grams of product require 4.0 L of sulphuric acid
Asked : 8.0 grams of product require how many liters of same sulphuric acid
Thus using Unitary method:
3.2 grams of product require sulphuric acid = 4.0 Liters
Thus 8.0 grams of product require sulphuric acid =