Answer:
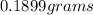
Explanations:
The balanced chemical reaction between hydrochloric acid and potassium sulfide is as shown:
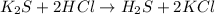
Based on stoichiometry, we can see that 1 mole of potassium sulfide reacted to form 1 mole of hydrogen sulfide.
Get the mole of hydrogen sulfide gas (H2S) using the ideal gas equation expressed as:
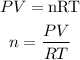
P is the pressure of the gas (in atm) = 0.994737atm (756torr)
V is the volume of the gas = 42.5mL = 0.0425L
T is the temperature (in Kelvin) = 26 + 273 = 299K
R is the gas constant = 0.0821 L*atm/mole * K
Substitute these values into the formula as shown:

Since the number of moles of hydrogen sulfide is 0.00172moles, the number of moles of potassium sulfide will also be 0.00172 moles (based on stoichiometry)
Get the mass of potassium sulfide that reacted using the formula:
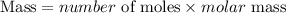
Number of moles of K2S = 0.00172 moles
Molar mass of K2S = 110.262 g/mol
Substitute into the formula for calculating the mass;
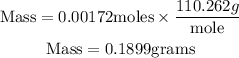
Therefore the mass of potassium sulfide that reacted (in grams) is approximately 0.1899grams