Answer : The correct option is,
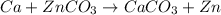
Explanation :
Displacement reaction : It is a chemical reaction in which a most reactive element displaces the least reactive element easily from its compound.
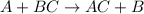
In this, A is more reactive element and B is less reactive element.
The given balanced chemical reaction will be,
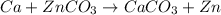
In this reaction, calcium displace the zinc element easily because calcium is more reactive element than the zinc.