Answer: Option (c) is the correct answer.
Step-by-step explanation:
As density is mass divided by volume.
Mathematically, Density =
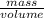
So, more is the mass of a substance more will be its density but if volume is low then density will also have a low value.
Molar mass of neon is 20 g/mol.
Molar mass of
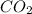 is 44 g/mol.
is 44 g/mol.
Molar mass of
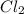 is 70.9 g/mol.
is 70.9 g/mol.
Since,
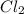 molecule has the highest mass. Therefore, its density will also be high.
molecule has the highest mass. Therefore, its density will also be high.
Thus, we can conclude that out of the given options
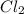 gas has the highest density.
gas has the highest density.