Answer:
0.5 M is the concentration of NaOH used.
Step-by-step explanation:
Considering:
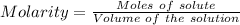
Or,
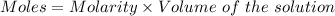
Given :
For
 :
:
Molarity = 2.00 M
Volume = 0.25 L
Thus, moles of
 :
:
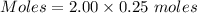
Moles of
 = 0.5 moles
= 0.5 moles
According to the given reaction:
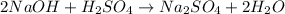
1 mole of
 reacts with 2 moles of
reacts with 2 moles of
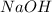
0.5 mole of
 reacts with 2*0.5 moles of
reacts with 2*0.5 moles of
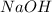
Moles of
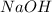 = 1.0 moles
= 1.0 moles
Given that volume of NaOH reacted = 2.00 L
So,
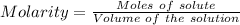
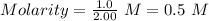
0.5 M is the concentration of NaOH used.