Answer: 8 moles of hydrogen reacts with 1 mole of sulfur.
Explanation: We are given a balanced chemical equation:
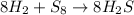
By Stoichiometry, it is said that
8 moles of Hydrogen gas reacts with 1 mole of sulfur to produce 8 moles of hydrogen sulfide.
Mole ratio of reactants = Hydrogen : Sulfur = 8 : 1