Step-by-step explanation:
The given compound is
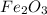 and it contains atoms of iron and oxygen.
and it contains atoms of iron and oxygen.
Number of atoms of different element present in the given formula are as follows.
Number of iron atoms = 2
Number of oxygen atoms = 3
Thus, total number of atoms present in
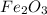 are as follows.
are as follows.
Total atoms present = number of Fe atoms + number of O atoms
= 2 + 3
= 5
Hence, we can conclude that there are total 5 atoms in a
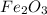 .
.