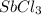Answer : The correct option is,
Explanation :
The symbol of antimony is 'Sb'.
The symbol of chlorine is 'Cl'
Covalent compound rules :
- First write down the symbol of the first element.
- Use the prefix to determine the atoms of first element. If there is no prefix on element then there is only 1 atom.
- Now write down the symbol of the second element.
- Use the prefix to determine the atoms of second element.
- Use prefix as 'mono' for '1', 'di' for '2', 'tri' for '3' and so on.
Therefore, the correct formula for antimony trichloride is