The theoretical yield of alum = 8.62 g
Explanation
Given:
Mass of aluminum that reacted = 0.49 g
Molar mass of aluminum = 26.98 g/mol
Molecular mass of alum = 474.38 g/mol
What to find:
The theoretical yield of alum.
Explanation:
If 0.46 g of aluminum reacted, then the number of moles of aluminum that reacted will be:
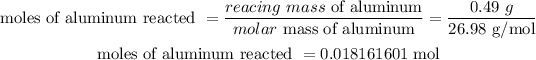
The molecular mass of alum = 474.38 g/mol
The chemical formula of alum is KAl(SO₄)₂.12H₂O
1 mol Al → KAl(SO₄)₂.12H₂O
Thus, the theoretical yield of alum is:
