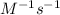Answer : The correct option is, (B)
Step-by-step explanation:
Rate of reaction : It is defined as the rate of change in concentration of reactant or product with respect to time.
The given rate expression is,
![Rate=k[A][B]](https://img.qammunity.org/2018/formulas/chemistry/high-school/sy3xqhzscgazgcufqhzovy5o69p5t6swsh.png)
From this expression we conclude that the power of concentration of reactant A and B are 1, 1. That means it is a second order reaction.
The formula for determining the unit of 'k' is:
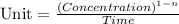
where, n = order of reaction
The unit of concentration is, M or mole/L
The unit of time is, second or 's'.
When n = 0
The unit of 'k' =
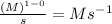
When n = 1
The unit of 'k' =
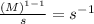
When n = 2
The unit of 'k' =
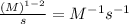
and so on.....
Therefore, the unit of 'k' of the following expression is,