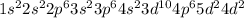Answer: The number of electrons in an atom of zirconium are 40.
Step-by-step explanation:
Electrons are one of the subatomic particles present in an atom.
Electrons in an atom is judged by the atomic number of the atom.
Atomic number = Number of protons = Number of electrons
Atomic number of Zirconium = 40
Number of electrons will be equal to 40
Electronic configuration of Zirconium (Z = 40):