Step-by-step explanation:
The equation for work done by a system is as follows.
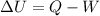
where,
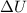 = change in internal energy
= change in internal energy
Q = heat absorbed or released by the system
W = work done by the system
Since it is given that it is an isothermal process. Therefore,
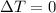 .
.
Whereas internal energy depends on temperature and as there is no change in temperature so, there will be no change in internal energy of the system.
Hence, the equation will be as follows.
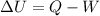
0 = Q - W
Q = W
Therefore, we can conclude that for an isothermal process, the work done by or on a system of ideal gas is equal to the change in Q.