Answer: The balanced chemical equation is written below.
Step-by-step explanation:
Every balanced chemical equation follows law of conservation of mass.
Law of conservation of mass states that mass can neither be created nor be destroyed but it can only be transformed from one form to another form.
This also means that total mass on the reactant side must be equal to the total mass on the product side.
The chemical equation for the reaction of sodium carbonate and calcium chloride follows:
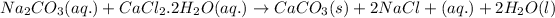
By Stoichiometry of the reaction:
1 mole of sodium carbonate reacts with 1 mole of calcium chloride dihydrate to produce 1 mole of calcium carbonate, 2 moles of sodium chloride and 2 moles of water.
Hence, the balanced chemical equation is written above.